
Contents
Introduction
There are several types of screening test available in pregnancy, which aim to identify significant foetal anomalies in the uterus, and thus provide the parents with the either an option to terminate the pregnancy, or advice and time to plan to cope with a child with a serious illness. These tests are typically performed in the first or second trimester, and collectively are sometimes referred to as prenatal testing.
In this article, we discuss the different types of screening, their indications and their pros and cons.
For more information about overall antenatal care, see Pregnancy – antenatal care.
There are five main types of antenatal screening test offered to women in Australia. Options are similar in the UK, but the exact service may vary by location and some of them may not be available on the NHS.
These are:
- Combined first trimester screening test
- The combination of USS and blood test typically offered between 11 and 14 weeks gestation
- Gives a probability score – e.g. 1 in 500 for the probability of various trisomies – including Down Syndrome
- Cell free DNA screening aka Non-invasive prenatal testing (NIPT)
- A maternal blood test which tests for traces of foetal DNA. Is also able to detect the gender of the child
- Gives a yes / no result if an abnormality is detected – but a positive result should be confirmed with invasive testing if the mother is considering termination
- Second trimester quadruple screening test
- Similar to the combined first trimester screening test, but also including additional factors in the blood test. Useful for those who have missed the combined first trimester screening
- USS is not ‘officially’ part of the ‘quadruple’ part of the test but is also often performed
- Chorionic villus sampling – CVS
- A sample is taken via needle from the placenta via USS guidance
- Used to confirm high risk or positive results from earlier screening tests
- Amniocentesis
- A sample is taken via needle from the amniotic fluid under USS guidance
- Used to confirm high risk or positive results from earlier screening tests
Women my choose to have one, a combination or none of these tests during their pregnancy. Some of these tests have a time-frame during which they must be performed.
Generally speaking, in both Australia and the UK, almost all women will opt for a combined first trimester screening test and some (in increasing numbers as the cost comes down) will also opt for a NIPT privately – which as of October 2020, costs about A$400 or £350.
Most women will have a combined first trimester screening test, and if this is normal, no further testing is routinely recommended. NIPT testing is becoming increasingly popular. It provides greater accuracy for some fatal anomalies than the combined screening test, but also the lack of an USS means it is not able to detect other potential abnormalities. It is generally recommended as a complementary test in additional to the combined test, rather than instead of it, in women who choose to have it (and pay for it).
The quadruple screening test is generally reserved for those who have missed the earlier testing, and CVS and amniocentesis are reserved for high risk cases (either determined by maternal age, or by test results from earlier screening).
Combined first trimester screening test (CFTS)
- This is the most commonly performed pregnancy screening test
- It should be offered to all pregnant women
- Can be performed between 9 weeks (some centres say 11 weeks and 1 day) gestation and 13 weeks and 6 days gestation
- If dates are uncertain, you could consider a dating USS earlier in the pregnancy to confirm the dates
- Calculates a risk score for the following fatal anomalies:
- Trisomy 21 – Down Syndrome
- Trisomy 18 – Edward Syndrome
- Trisomy 13 – Patau Syndrome
- Patau and Down syndrome are not typically compatible with life. They may result in late miscarriage, stillbirth or a neonate that survives for only a very brief period
- This risk score is given as an individual figure for each trisomy – e.g. 1 in 5,000 risk
- Different centres have different cut-offs for what is deemed to be “high risk”. This is typically in the 1 in 200 or 1 in 300 range
- Mothers deemed to be at high risk will be offered definitive testing, such as CVS or amniocentesis
- Maternal age is also important in determining the pre-test probability
- Rising maternal age is associated with an increased risk of Down Syndrome
- The background risk is about 1 in 500
- By the age of 35, this is about 1 in 350, and by age 40 this is about 1 in 100
- As such, in women aged in the late 30s and older, CVS or amniocentesis may be offered as an initial test (as the risks are lower than the risk of the pregnancy), or women may wish to have their own personalised risk score first from the combined screening test or NIPT, before considering CVS or amniocentesis
- The combined first trimester screening test detects about 90% of cases of Down Syndrome
The test itself involves:
- B-hCG (blood)
- PAPP-A (blood)
- Nuchal translucency – NT (USS)
Typically both of these are performed on the same day – but some local protocols may vary.
Using a formula (online calculators are available), values from these tests can be used to determine a risk score for the foetus for several genetic anomalies. This is normally calculated by the lab performing the test, and the requesting doctor will usually be given the result without having to perform the calculation.
As well as being useful in deterring the risk for trisomies, the PAPP-A and Nuchal Translucency results can be used independently as risk factors for other diseases.
PAPP-A
- PAPP-A is a biochemical marker found in pregnancy, which stands for Pregnancy Associated Plasma Protein – A
- It is produced by the placenta and is thought to have several roles, including preventing the foetus being detected by the maternal immune system
- A low PAPP-A is associated with poor early development of the placenta, with various increased risks in pregnancy, including increased risk of eclampsia, fatal growth restriction, pre-term birth and increased risk of miscarriage
- Results are given with the units MoM standing for “multiples of the mean”
- An abnormal result is PAPP-A <0.4 MoM (this is a result below the 5th percentile)
- There is no association between a high level and adverse pregnancy outcomes
- Some argue that those with a low PAPP-A should be monitored with USS regularly throughout pregnancy
Nuchal Translucency (NT)
Nuchal translucency is a measure of the amount of fluid in back of the neck in the developing foetus.
An increase in nuchal translucency is a sign of oedema in the foetus, which is correlated with various congenital abnormalities. Therefore increased nuchal translucency is a poor prognostic factor.
- Foetuses with a nuchal translucency of >3.5mm (>95th percentile) are deemed to be at increased risk
- This risk rises exponentially as the risk increases
- It is recommended that babies with an increased nuchal translucency undergo further ultrasound scanning at laster gestations for detection of abnormalities
- Nuchal translucency needs to be measured between 11 and 14 weeks gestation – after this time, development of the foetus means that nuchal translucency can no longer be accurately measured
Cell free DNA screening aka NIPT
This is a blood test for the mother that tests for foetal fragments of DNA. These fatal fragments can then be used to determine if trisomies are present and can determine the gender of the baby. In Australia it is not covered by Medicare and costs about $200-300.
- In Australia, the test is also known to patients as the Harmony test – named after a branded version of the test
NIPT tests for the same three aneuploidies at the CFTS (13, 18 and 21). In addition, it can also tests for other genetic abnormalities – and these will vary by the the lab that provides the test. However, NIPT does not test for single gene syndromes (e.g. cystic fibrosis) – only for chromosomal abnormalities.
NIPT is a relatively newer form of testing, and exactly where it fits into the screening pathway can seem a little vague and complicated. Although NIPT tests for common trisomies (like the combined first trimester screening test), it is not recommended to replace the combined first trimester screening test. This is because the combined test also includes other valuable information in the blood and USS test that can affect recommended follow-up care (see PAPP-A and NT above). Many women choose to have both tests, and those with an increased risk on the combined test may subsequently choose to have NIPT testing performed.
NIPT has 99% specificity and lower false positive rate than the triple and quadruple tests. The fast positive rate is quoted at around 0.4%. However, there is a failure rate of about 1% – when not enough fatal DNA is available to isolate from the mother’s DNA.
The tests looks for cfDNA (cell-free DNA):
- This is fragments of DNA outside of cells
- It is typically cleared quickly from the blood stream
- In the NIPT we are looking for cfDNA from the placenta – which represents the genotype of the foetus
- A NIPT requires about 10% of the cfDNA to be from the foetus. This percentage rises as the pregnancy proceeds – from about 10 weeks, the percentage is usually >10%.
- A low foetal fraction is more likely in patients with a high BMI
- A low foetal fraction results in a ‘failed test’ which affects between about 2-6% of tests
Whereas the combined screening test will give a risk score the NIPT test will give an answer of more certainty, e.g. “Abnormality not detected” vs “Abnormality detected” listed next to each test.
- NIPT is especially accurate at detecting Down Syndrome
In the case of a positive test – it is still recommended that if parents are considering termination of pregnancy they undergo a confirmatory test such as CVS or amniocentesis.
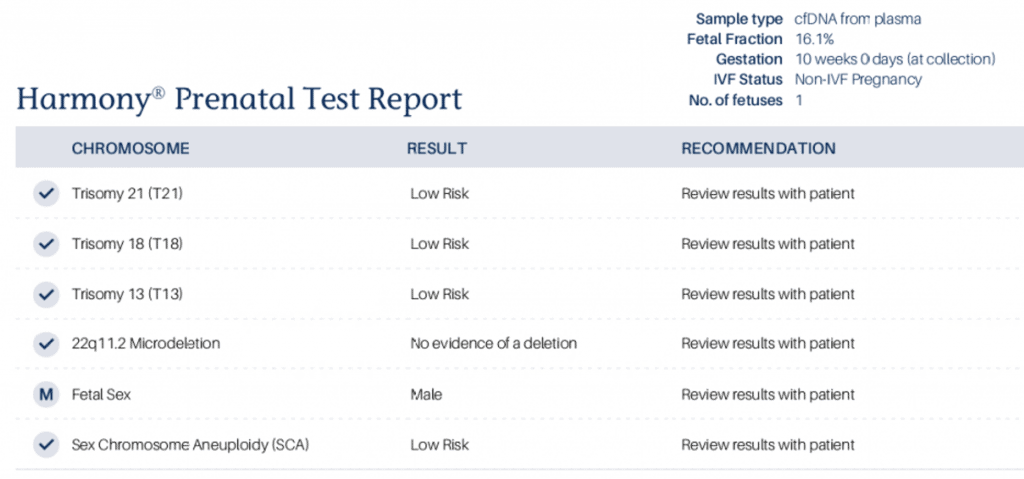
An example of a normal NIPT test result. Note that in this brand of test, the result is given as “low risk”. Other wording I have seen has included “no abnormality detected” or “no aneuploidy detected”.
Comparing CFTS and NIPT
| CFTS | NIPT | |
|---|---|---|
| Dates | 11+1 to 13+6 | From 10 weeks onwards |
| Detection rate | 85-90% | 99% |
| False positive rate | 4% | 0.4%
|
| Other benefits | PAPP-A and NT can both be indicators of other foetal abnormalities and health and may alter ongoing monitoring recommendations in pregnancy | Nil |
| Cost | Covered by NHS in UK. Typically A$100-200 in Australia | Typically £350 or A$300-400 |
Even with a positive NIPT test – invasive pre-natal testing (amniocentesis or CVS) is still recommended to confirm the diagnosis if a patient is considering termination.
Quadruple Test
The second trimester quadruple screening test is useful for pregnancies that present in the second trimester and may have missed the window for the combined first trimester screening test.
It can be performed between 14 and 20 weeks.
- Alpha FP
- Unconjugated estradiol
- BetaHCG
- Inhibin A – not widely available in the UK
It is also often accompanied by a fatal anomaly ultrasound scan, but this is not technically part of the “quadruple” test. The test, like a combined first trimester screen, given a risk score – i.e. “1 in xxx'” chance – and high risk women will be offered further screening via CVS or amniocentesis.
Invasive prenatal testing
Both CVS and amniocentesis are sometimes collectively referred to as invasive prenatal testing. About 5% of pregnancies will be offered some form of invasive prenatal testing. These test should be performed by specialists with experience in doing these procedures, as this may be associated with a lower fatal loss rate than less experienced providers.
Amniocentesis is the most common form of invasive prenatal testing.
Chorionic Villus Sampling (CVS)
- A sample of the placenta is taken via an USS guided needle
- Can be done transvaginally or transabdominally
- Can be performed between 11-14 weeks
- Carries an approximate risk of fatal loss of about 1%
- Newer studies suggest this risk may actually be much lower
Amniocentesis
- A sample of amniotic fluid is taken via an USS guided needle
- Can be performed from 15 weeks gestation
- Amniocentesis before 15 weeks is associated with a higher foetal loss rate
- Risk of foetal loss is about 0.5% (slightly safer than CVS)
- Newer studies suggest this risk may actually be much lower





